Enterovirus 71 (EV71)-IgM Detection Kit
【INTENDED USE】
Enterovirus 71 (EV71)-IgM Detection Kit (Colloidal Gold Method) is a lateral flow immunoassay for the qualitative detection of IgM-class antibodies to human Enterovirus 71 (EV71) in serum, plasma or whole blood samples. It is intended to be used as a screening test and provides a preliminary test result for early diagnosis and management of patients related to infection with EV71.
【TEST PRINCIPLE】
This kit adopts colloidal gold-immunochromatography assay (GICA).
The test card contains:
1. Colloidal gold-labeled antigen and quality control antibody complex.
2. Nitrocellulose membranes immobilized with one test line (T line) and one quality control line (C line).
When an appropriate amount of sample is added to the sample well of the test card, the sample will move forward along the test card under capillary action.
If the sample contains an IgM antibody of EV71, the antibody will bind to the colloidal gold-labeled EV71 antigen, and the immune complex will be captured by the monoclonal anti-human IgM antibody immobilized on the nitrocellulose membrane to form a purple/red T line , showing that the sample is positive for IgM antibody.
【Reagents And Materials Supplied】
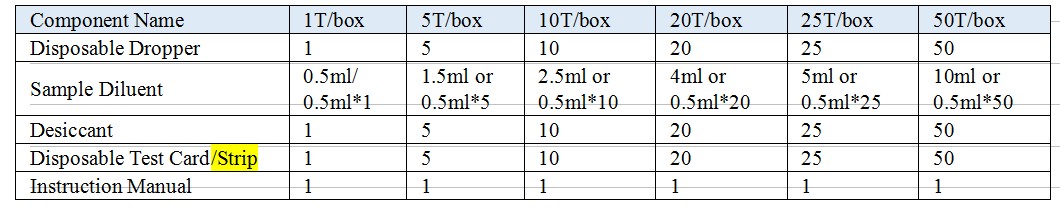
Model: Test Card, Test Strip
【SHELF LIFE AND STORAGE】
1. The original packaging should be stored in a dry place at 2-30°C and protected from light.
2. The shelf life of the test kit is 2 years from date of manufacture. Refer to the product labels for stated expiration date.
3. The original packaging can be transported at 2-37℃ for 20 days.
4. After opening the inner package, the test card will become invalid due to moisture absorption, please use it within 1 hour.
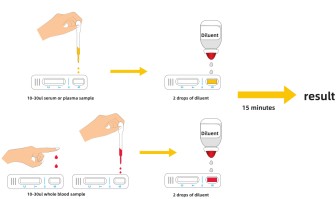
【Test procedure】
Step1: Allow the test device, buffer, specimen to equilibrate to room temperature (15-30℃) prior to testing.
Step2: Remove the test device from the sealed pouch. Place the test device on a clean, flat surface.
Step3: Label the device with specimen number.
Step4: Using a Disposable Dropper, transfer serum, plasma or whole blood. Hold the dropper vertically and transfer 1 drop of specimen (approximately 10μl) to the specimen well(S) of the test device, and immediately add 2 drops of test buffer (approximately 70-100μl). Make sure there are no air bubbles.
Step5: Set up a timer. Read the results in 15 minutes.
Do not interpret the result after 20 minutes. To avoid confusion, discard the test device after interpreting the result. If you need to store it for a long time, please take a photo of the result.
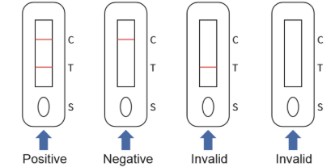
【INTERPRETATION OF ASSAY RESULT】
NEGATIVE:
If only the quality control line C appears, and the test lines T is not purple/red, it indicates that no antibody is detected, and the result is negative.
POSITIVE:
If both the quality control line C and the test line T appear purple/red, it indicates that the IgM antibody is detected, and the result is positive for IgM antibody.
INVALID:
If the quality control line C is not displayed, the test result is invalid regardless of whether there is a purple/red test line, and it should be tested again.